ChronoSteril
Real time visibility of your instrument sterilization process
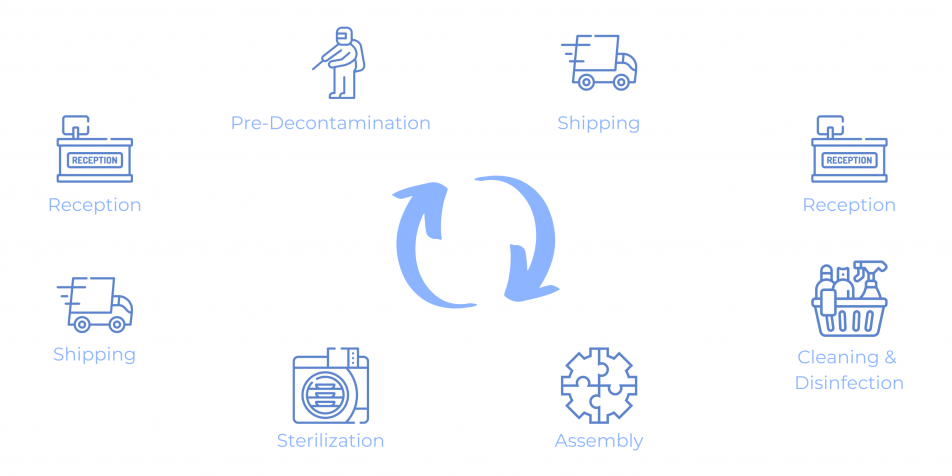
Accessible on any platform, with any interface
An overview of our software, that is flexible to your needs and allows real-time data
entry analysis.
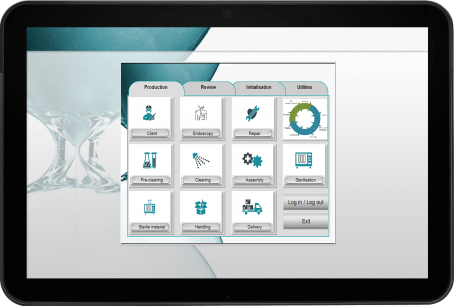
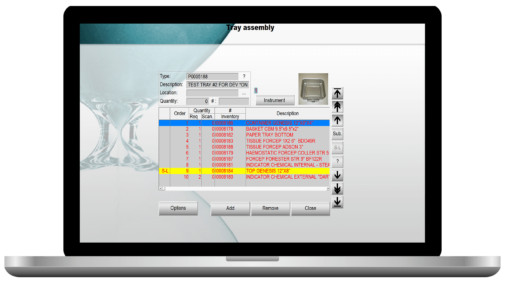
Manage your whole tray assembly process
Tray's compliances: quality assurance, infection control, content order & positioning of medical devices.
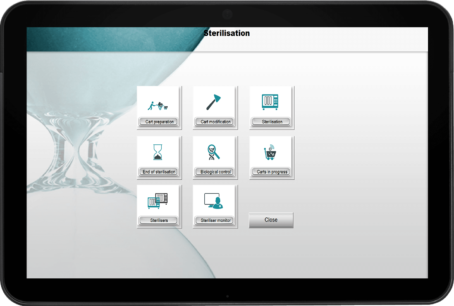
Track your entire sterilization cycle
Ensures that every instrument has been properly sterilized, with references and instructions for each instrument and sterilizer.
Quality Recall
Receive a complete sterilization Load Record that includes sterilizer operator, sterilizer identifier, lot number, date-time stamp, and all load contents.
Quickly locate affected sets to pull them from use, proactively preventing the spread of infection assuring the sets are not used on patients.
Unique Identifiers
Verify that instrument sets are correctly assembled, sterilized, decontaminated, maintained and stored through constant QA checks and thorough records throughout the lifecycle process.
Access your records at any time in the event of a quality breakdown to pinpoint the origin of the outbreak. Enables accountability throughout the lifecycle process.
Quality Assurance
Ensure that even the newest employees have accurate technical knowledge with complete assembly instructions and photos of both completed sets and individual instruments.
Receive an electronic audit trail that details exactly where instruments have been used and how they have been processed.
Biological Indicators
Record biological indicators to provide a Quality Assurance trail of the sterilization process. Provides a timing fail safe to ensure biologicals are read when they are due to be read.
Record stat decontamination and flash sterilization activity to give you a history of set and equipment flash sterilization activity.
